Iron substitution in Na4VMn(PO4)3 as a strategy for improving the electrochemical performance of sodium-ion batteries - ScienceDirect
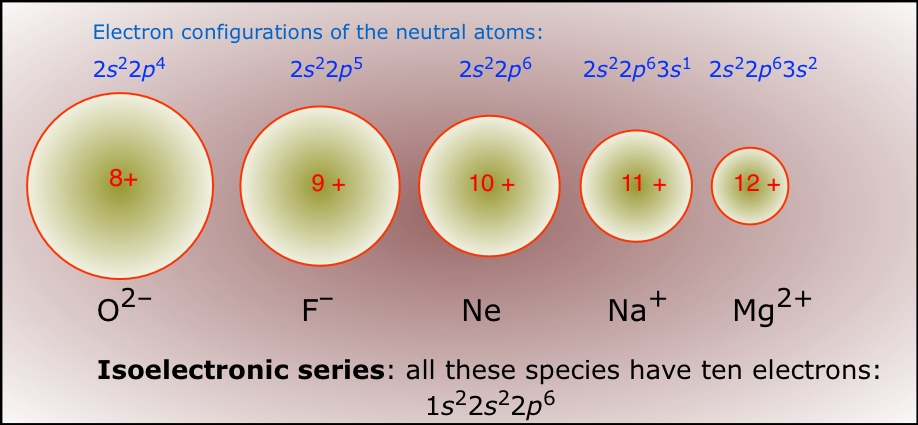
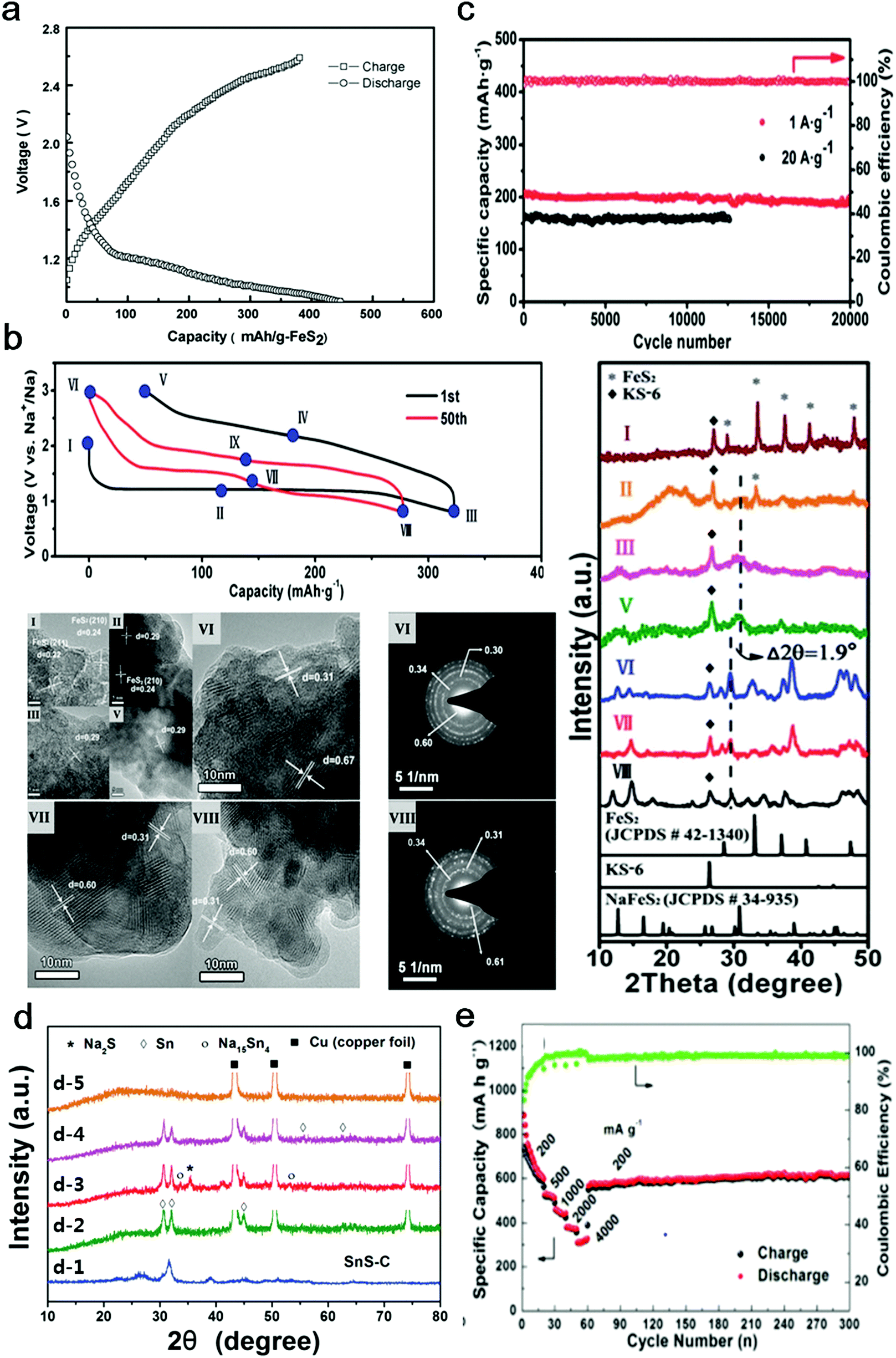
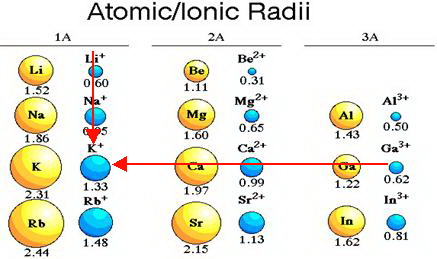
The primary reason sodium ions are smaller than sodium atoms is that the ion has only two shells of electrons (the atom has three). Some resources suggest the ion gets smaller since

The relationship between the size of the ions in the ionic liquid and... | Download Scientific Diagram

Schematic structure and estimated size of simple metal cations like... | Download Scientific Diagram

6.3 Periodic Trends Sodium chloride (table salt) produced the geometric pattern in the photograph. Such a pattern can be used to calculate the position. - ppt video online download

Revealing sodium-ion diffusion in alluaudite-type Na4–2xM1+x(MoO4)3 (M = Mg, Zn, Cd) from 23Na MAS NMR and ab initio studies - ScienceDirect
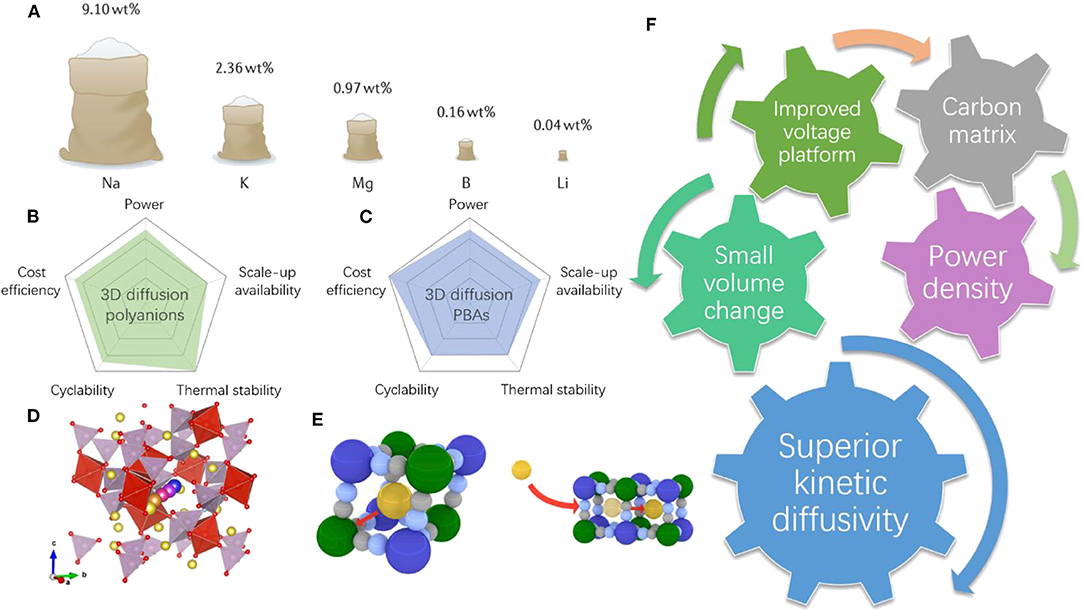
Frontiers | Building High Power Density of Sodium-Ion Batteries: Importance of Multidimensional Diffusion Pathways in Cathode Materials | Chemistry

PDF) Nanometer-size Na cluster formation in micropore of hard carbon as origin of higher-capacity Na-ion battery