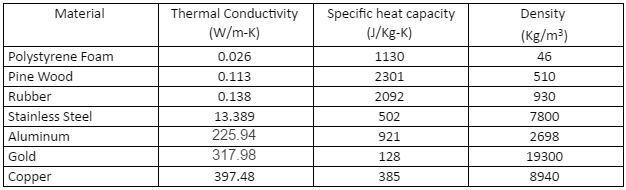
Change in the specific heat capacity of the wood c depending on t and u | Download Scientific Diagram

Variation of specific heat capacity of wood species for dry wood and... | Download Scientific Diagram
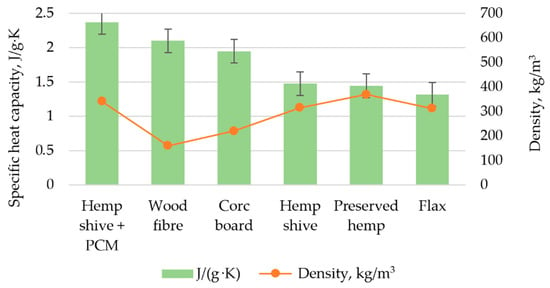
Buildings | Free Full-Text | Experimental Study of Using Micro-Encapsulated Phase-Change Material Integrated into Hemp Shive Wallboard | HTML

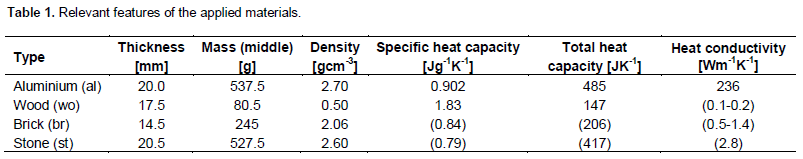
Table 1 from Potential for Hygroscopic Building Materials to Improve Indoor Comfort and Air Quality in the Canadian Climate | Semantic Scholar

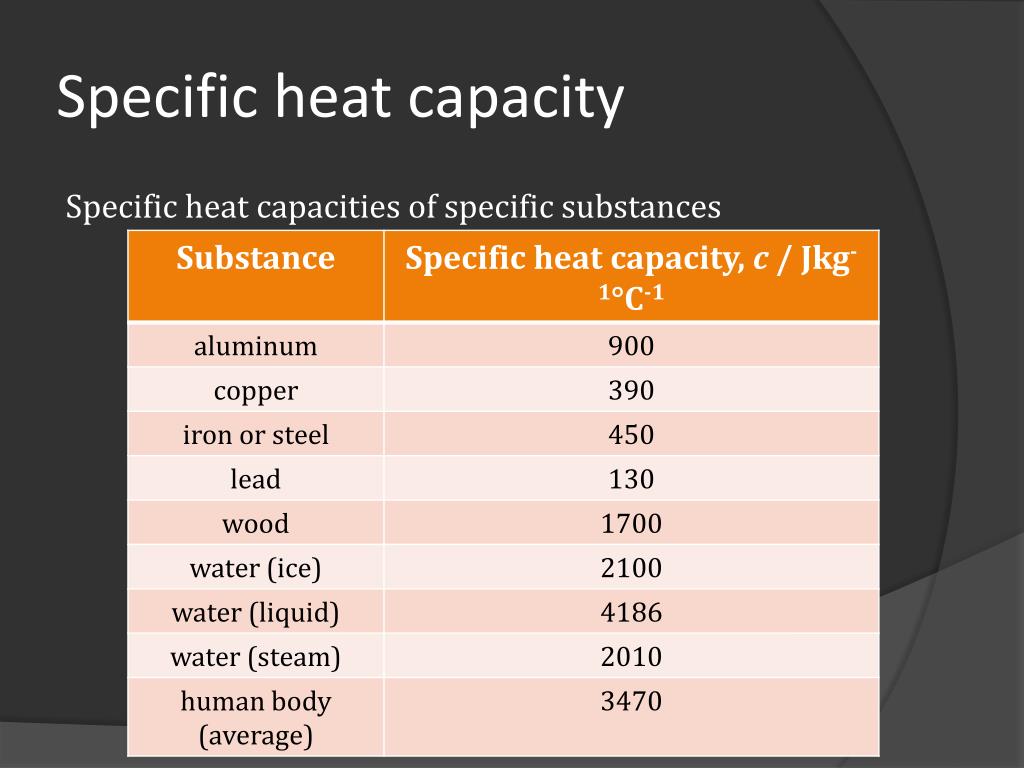
SOLVED:1_ To a 1 kg sample of wood 40kJ of heat is added, and its temperature is found to rise from 20 to 449. What is the specific heat capacity of the
International Journal of Science and Technology Education Research - the solar-reflective characterization of solid opaque materials

⚗️A piece of wood near a fire is at 23°C. It gains 1,160 joules of heat from the fire and reaches a - Brainly.com



![PDF] Specific Heat Capacity of Wood | Semantic Scholar PDF] Specific Heat Capacity of Wood | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b5a6b9fa21024174fb849f496faf271b114afc67/2-Table1-1.png)






![PDF] Specific Heat Capacity of Wood | Semantic Scholar PDF] Specific Heat Capacity of Wood | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b5a6b9fa21024174fb849f496faf271b114afc67/3-Figure1-1.png)